“The Role of the Tumor Microenvironment in Leukemia Progression
Related Articles The Role of the Tumor Microenvironment in Leukemia Progression
- Innovations In Medical Devices For Chronic Disease Management
- The Role Of Genetics In Chronic Disease Development – Part 2
- Integrative Care Models For Complex Chronic Diseases
- Integrative Medicine In Chronic Disease Care – Part 3: Specific Modalities And Their Application
- Integrative Care Models For Complex Chronic Diseases – Part 7: The Role Of Technology In Enhancing Integrative Care Delivery
Introduction
On this special occasion, we are happy to review interesting topics related to The Role of the Tumor Microenvironment in Leukemia Progression. Come on knit interesting information and provide new insights to readers.
Table of Content
The Role of the Tumor Microenvironment in Leukemia Progression

Leukemia, a hematological malignancy characterized by the uncontrolled proliferation of abnormal blood cells in the bone marrow, is not solely driven by the intrinsic genetic and epigenetic aberrations within the leukemic cells themselves. The tumor microenvironment (TME), a complex ecosystem surrounding the leukemic cells, plays a crucial and multifaceted role in leukemia initiation, progression, drug resistance, and relapse. This article delves into the intricate interplay between leukemic cells and the TME, highlighting the key cellular and non-cellular components of the TME and their contributions to leukemia pathogenesis.
I. The Tumor Microenvironment: A Complex Ecosystem
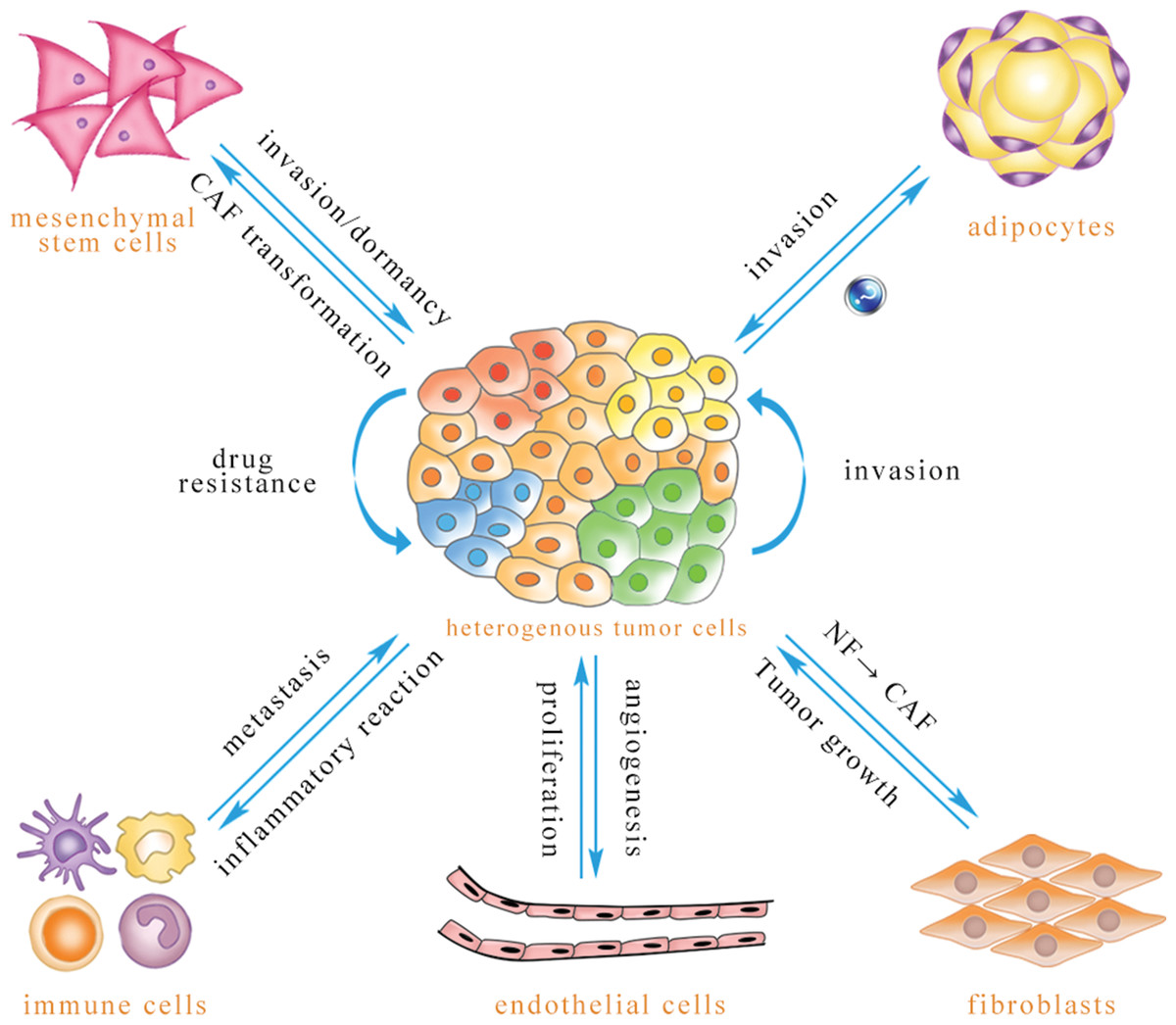
The TME in leukemia encompasses a diverse array of cellular and non-cellular components that interact dynamically with leukemic cells. These components include:
- Bone Marrow Stromal Cells (BMSCs): BMSCs, including mesenchymal stromal cells (MSCs), endothelial cells, osteoblasts, and adipocytes, provide structural support and secrete various growth factors, cytokines, and chemokines that influence leukemic cell survival, proliferation, and differentiation.
- Immune Cells: Immune cells, such as T cells, B cells, natural killer (NK) cells, macrophages, and dendritic cells (DCs), infiltrate the bone marrow and can either promote or suppress leukemia progression. The balance between anti-tumor immunity and immune suppression within the TME is critical in determining the fate of leukemic cells.
- Extracellular Matrix (ECM): The ECM, a complex network of proteins, glycoproteins, and proteoglycans, provides structural support and regulates cell adhesion, migration, and signaling. The ECM in the leukemia TME is often remodeled, creating a niche that favors leukemic cell survival and proliferation.
- Soluble Factors: Soluble factors, including growth factors (e.g., stem cell factor [SCF], FLT3 ligand), cytokines (e.g., interleukin-6 [IL-6], tumor necrosis factor-alpha [TNF-α]), chemokines (e.g., CXCL12, CCL3), and exosomes, mediate communication between leukemic cells and the TME, influencing various aspects of leukemia biology.
- Hypoxia and Metabolic Stress: Hypoxia, or low oxygen tension, is a common feature of the leukemia TME, particularly in densely populated areas of the bone marrow. Hypoxia induces metabolic stress in leukemic cells, leading to the activation of adaptive mechanisms that promote survival and drug resistance.
II. The Role of Bone Marrow Stromal Cells in Leukemia
BMSCs play a pivotal role in supporting leukemic cell survival, proliferation, and drug resistance.
- Survival and Proliferation: BMSCs secrete growth factors and cytokines, such as SCF and IL-6, that stimulate the proliferation and survival of leukemic cells. MSCs, in particular, provide a protective niche for leukemic cells, shielding them from chemotherapy-induced apoptosis.
- Drug Resistance: BMSCs can confer drug resistance to leukemic cells through various mechanisms, including:
- Cell Adhesion-Mediated Drug Resistance (CAM-DR): Adhesion of leukemic cells to BMSCs via adhesion molecules like VLA-4 and fibronectin triggers intracellular signaling pathways that inhibit apoptosis and promote drug resistance.
- Cytokine-Mediated Drug Resistance: BMSCs secrete cytokines that activate survival pathways in leukemic cells, rendering them less sensitive to chemotherapy.
- Exosome-Mediated Drug Resistance: BMSCs release exosomes containing microRNAs (miRNAs) and other factors that can transfer drug resistance to leukemic cells.
- Leukemia Stem Cell (LSC) Maintenance: LSCs, a subpopulation of leukemic cells with self-renewal and differentiation capabilities, are responsible for leukemia initiation, relapse, and drug resistance. BMSCs provide a niche that supports LSC survival and self-renewal, contributing to the persistence of leukemia.
III. The Immune System in Leukemia: A Double-Edged Sword
The immune system plays a complex and often paradoxical role in leukemia. While immune cells can recognize and eliminate leukemic cells, they can also be co-opted by leukemic cells to promote their survival and proliferation.
- Anti-Tumor Immunity: T cells, NK cells, and DCs can recognize and kill leukemic cells through various mechanisms, including:
- Cytotoxic T Lymphocyte (CTL)-Mediated Killing: CTLs recognize leukemia-associated antigens (LAAs) presented on the surface of leukemic cells and kill them through the release of cytotoxic granules.
- NK Cell-Mediated Killing: NK cells kill leukemic cells that have lost expression of MHC class I molecules or express stress-induced ligands.
- DC-Mediated Antigen Presentation: DCs capture and process LAAs and present them to T cells, initiating an anti-leukemia immune response.
- Immune Suppression: Leukemic cells can evade immune surveillance and suppress anti-tumor immunity through various mechanisms, including:
- Expression of Immune Checkpoint Molecules: Leukemic cells express immune checkpoint molecules, such as PD-L1, which bind to inhibitory receptors on T cells, suppressing their activation and effector functions.
- Secretion of Immunosuppressive Cytokines: Leukemic cells secrete immunosuppressive cytokines, such as IL-10 and TGF-β, which inhibit T cell activation and promote the development of regulatory T cells (Tregs).
- Recruitment of Myeloid-Derived Suppressor Cells (MDSCs): Leukemic cells recruit MDSCs to the TME, where they suppress T cell activity and promote immune tolerance.
- Exosome-Mediated Immune Suppression: Leukemic cells release exosomes containing immunosuppressive molecules, such as miRNAs and proteins, that inhibit T cell function.
IV. The Extracellular Matrix in Leukemia
The ECM in the leukemia TME is often remodeled, creating a niche that favors leukemic cell survival and proliferation.
- ECM Remodeling: Leukemic cells and BMSCs secrete enzymes, such as matrix metalloproteinases (MMPs), that degrade and remodel the ECM. This remodeling creates space for leukemic cell expansion and facilitates their migration and invasion.
- Cell Adhesion and Migration: The ECM provides adhesion sites for leukemic cells, allowing them to adhere to BMSCs and other components of the TME. This adhesion promotes cell survival and drug resistance. The ECM also guides leukemic cell migration, allowing them to disseminate to other sites in the body.
- Signaling: The ECM interacts with cell surface receptors, such as integrins, to activate intracellular signaling pathways that regulate cell survival, proliferation, and differentiation.
V. Soluble Factors in Leukemia
Soluble factors, including growth factors, cytokines, and chemokines, mediate communication between leukemic cells and the TME, influencing various aspects of leukemia biology.
- Growth Factors: Growth factors, such as SCF and FLT3 ligand, stimulate the proliferation and survival of leukemic cells.
- Cytokines: Cytokines, such as IL-6 and TNF-α, promote leukemic cell survival, proliferation, and drug resistance. They also regulate the recruitment and activation of immune cells.
- Chemokines: Chemokines, such as CXCL12 and CCL3, attract leukemic cells to the bone marrow and promote their adhesion to BMSCs. They also regulate the migration of immune cells.
- Exosomes: Exosomes, small vesicles secreted by cells, mediate the transfer of proteins, RNAs, and other molecules between leukemic cells and the TME. Exosomes can promote leukemic cell survival, drug resistance, and immune suppression.
VI. Hypoxia and Metabolic Stress in Leukemia
Hypoxia and metabolic stress are common features of the leukemia TME, particularly in densely populated areas of the bone marrow.
- Hypoxia-Inducible Factor-1 (HIF-1): Hypoxia activates HIF-1, a transcription factor that regulates the expression of genes involved in angiogenesis, glucose metabolism, and cell survival. HIF-1 promotes leukemic cell survival and proliferation under hypoxic conditions.
- Metabolic Adaptation: Leukemic cells adapt to metabolic stress by altering their metabolism, such as increasing glycolysis and glutaminolysis. These metabolic adaptations promote cell survival and proliferation.
- Drug Resistance: Hypoxia and metabolic stress can confer drug resistance to leukemic cells by activating survival pathways and inhibiting apoptosis.
VII. Therapeutic Implications
Targeting the TME represents a promising strategy for improving leukemia therapy.
- Targeting BMSCs: Strategies to disrupt the interaction between leukemic cells and BMSCs, such as inhibiting adhesion molecules or blocking cytokine signaling, can enhance the efficacy of chemotherapy.
- Modulating the Immune System: Immunotherapeutic approaches, such as checkpoint inhibitors and CAR T-cell therapy, can enhance anti-tumor immunity and eradicate leukemic cells.
- Targeting the ECM: Inhibiting ECM remodeling or blocking ECM-cell interactions can disrupt the leukemia niche and inhibit leukemic cell survival and migration.
- Targeting Soluble Factors: Blocking the action of growth factors, cytokines, or chemokines can inhibit leukemic cell proliferation and survival.
- Targeting Hypoxia and Metabolic Stress: Inhibiting HIF-1 or targeting metabolic pathways can disrupt leukemic cell survival and drug resistance.
VIII. Conclusion
The TME plays a critical role in leukemia progression, influencing leukemic cell survival, proliferation, drug resistance, and relapse. Understanding the complex interplay between leukemic cells and the TME is essential for developing novel therapeutic strategies that target the TME and improve outcomes for patients with leukemia. Targeting the TME, in combination with conventional chemotherapy or targeted therapies, holds great promise for eradicating leukemic cells and preventing relapse. Further research is needed to fully elucidate the mechanisms by which the TME influences leukemia biology and to develop effective TME-targeted therapies.







Leave a Reply