“The Role of Stress in Chronic Disease Progression – Part 9: The Gut-Brain Axis and the Modulation of Chronic Inflammation
Related Articles The Role of Stress in Chronic Disease Progression – Part 9: The Gut-Brain Axis and the Modulation of Chronic Inflammation
- Public Policy And Chronic Disease Prevention Strategies – Part 5: Policy Implementation, Evaluation, And Sustainability
- The Profound Impact Of Chronic Illness On Mental Health
- Nutritional Therapy For Chronic Disease Prevention – Part 6
- Exercise And Physical Activity Guidelines For Chronic Illness Management – Part 5: Mental Health Conditions (Depression And Anxiety)
- The Impact Of Chronic Illness On Mental Health – Part 7
Introduction
We will be happy to explore interesting topics related to The Role of Stress in Chronic Disease Progression – Part 9: The Gut-Brain Axis and the Modulation of Chronic Inflammation. Let’s knit interesting information and provide new insights to readers.
Table of Content
The Role of Stress in Chronic Disease Progression – Part 9: The Gut-Brain Axis and the Modulation of Chronic Inflammation

The intricate interplay between stress and chronic disease progression is a multifaceted area of research. In this ninth installment, we delve into the critical role of the gut-brain axis (GBA) in modulating chronic inflammation, a key driver in many chronic diseases. The GBA, a bidirectional communication network connecting the central nervous system (CNS) and the gastrointestinal tract, has emerged as a pivotal player in the stress response and its subsequent impact on systemic inflammation.
Understanding the Gut-Brain Axis
The GBA is a complex system comprising neural, hormonal, and immunological pathways. The gut, often referred to as the "second brain," hosts a vast and diverse community of microorganisms known as the gut microbiota. This microbial ecosystem interacts with the host through various mechanisms, influencing brain function and behavior. Conversely, the brain exerts control over gut function, motility, secretion, and immune responses.
Key components of the GBA include:
-
The Vagus Nerve: This is the longest cranial nerve, providing a direct anatomical link between the brainstem and the gut. It transmits sensory information from the gut to the brain and carries motor commands from the brain to the gut, influencing gut motility, inflammation, and immune responses.
-
The Enteric Nervous System (ENS): Often called the "intrinsic nervous system of the gut," the ENS is a complex network of neurons embedded in the gut wall. It regulates gut motility, secretion, and local immune responses independently of the CNS, but it also communicates with the brain via the vagus nerve and other pathways.
-
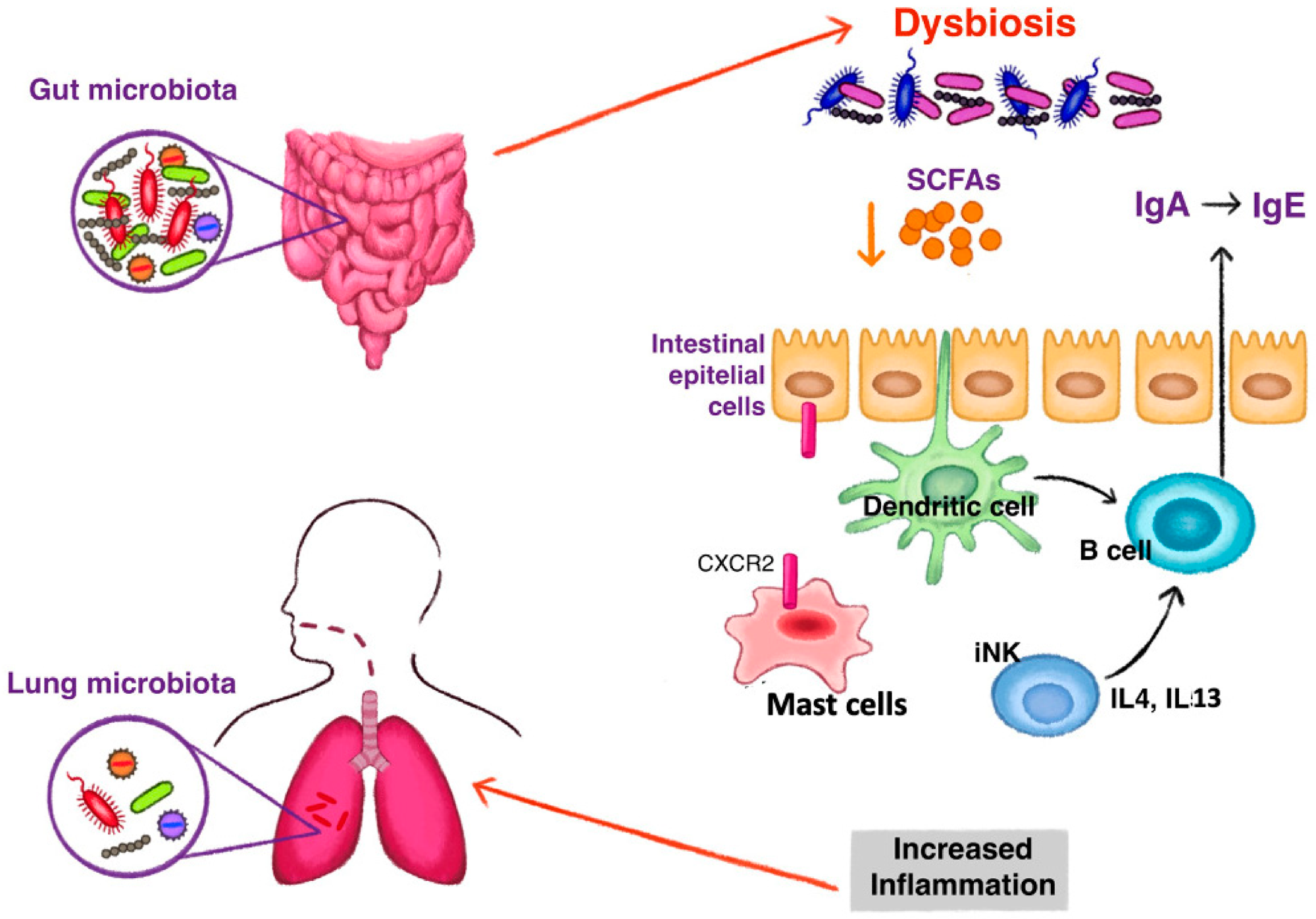
The Gut Microbiota: The trillions of microorganisms residing in the gut play a crucial role in digestion, nutrient absorption, and immune system development. They also produce a variety of metabolites, such as short-chain fatty acids (SCFAs), neurotransmitters, and other bioactive compounds that can influence brain function and behavior.
-
The Immune System: The gut-associated lymphoid tissue (GALT) is the largest immune organ in the body, housing a significant proportion of immune cells. The gut microbiota interacts with the GALT, shaping immune responses and maintaining a delicate balance between tolerance and immunity.
-
Neurotransmitters and Hormones: The gut and brain communicate through a variety of neurotransmitters and hormones, such as serotonin, dopamine, cortisol, and ghrelin. These signaling molecules can influence mood, appetite, stress responses, and immune function.
Stress and the Gut Microbiota
Stress, both acute and chronic, can significantly impact the composition and function of the gut microbiota. Studies have shown that stress can lead to:
-
Dysbiosis: An imbalance in the gut microbiota, characterized by a decrease in beneficial bacteria and an increase in pathogenic bacteria. Dysbiosis can disrupt gut barrier function, increase intestinal permeability ("leaky gut"), and promote inflammation.
-
Reduced Microbial Diversity: A decrease in the variety of microbial species in the gut. Lower diversity is associated with impaired immune function, increased susceptibility to infections, and chronic diseases.
-
Altered Microbial Metabolism: Changes in the production of microbial metabolites, such as SCFAs, which are important for gut health and immune regulation. Stress can reduce the production of beneficial metabolites and increase the production of harmful metabolites.
The mechanisms by which stress affects the gut microbiota are complex and involve:
- The Release of Stress Hormones: Cortisol, released during the stress response, can alter gut permeability, immune function, and microbial composition.
- Changes in Gut Motility: Stress can affect gut motility, leading to constipation or diarrhea, which can alter the gut environment and microbial composition.
- Altered Dietary Habits: Stress can influence dietary choices, leading to increased consumption of processed foods, sugar, and unhealthy fats, which can negatively impact the gut microbiota.
The Gut-Brain Axis and Chronic Inflammation
Chronic inflammation is a hallmark of many chronic diseases, including cardiovascular disease, diabetes, autoimmune disorders, and neurodegenerative diseases. The GBA plays a critical role in modulating chronic inflammation through several mechanisms:
-
Microbial Translocation: Dysbiosis and increased intestinal permeability can allow bacteria and their products, such as lipopolysaccharide (LPS), to translocate from the gut into the bloodstream. This triggers an immune response, leading to systemic inflammation.
-
Immune Activation: The gut microbiota can directly activate immune cells in the GALT, leading to the production of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. These cytokines can enter the circulation and contribute to systemic inflammation.
-
Vagal Nerve Signaling: The vagus nerve can modulate immune responses in the gut and systemically. Vagal nerve stimulation has been shown to reduce inflammation by inhibiting the production of pro-inflammatory cytokines and promoting the production of anti-inflammatory cytokines.
-
Microbial Metabolites: Microbial metabolites, such as SCFAs, can influence immune function and inflammation. SCFAs, particularly butyrate, have anti-inflammatory effects by inhibiting histone deacetylases (HDACs) and promoting the differentiation of regulatory T cells (Tregs).
Implications for Chronic Disease Progression
The GBA’s role in modulating chronic inflammation has significant implications for the progression of chronic diseases. Stress-induced dysbiosis and increased intestinal permeability can exacerbate chronic inflammation, contributing to the pathogenesis and progression of various diseases.
-
Cardiovascular Disease: Chronic inflammation is a key driver of atherosclerosis, the underlying cause of cardiovascular disease. Stress-induced dysbiosis and microbial translocation can promote inflammation in the arteries, accelerating the development of plaques.
-
Diabetes: Chronic inflammation contributes to insulin resistance and pancreatic beta-cell dysfunction in type 2 diabetes. Stress-induced dysbiosis can promote inflammation in the liver and adipose tissue, impairing insulin signaling and glucose metabolism.
-
Autoimmune Disorders: Autoimmune disorders, such as rheumatoid arthritis and multiple sclerosis, are characterized by chronic inflammation and immune dysregulation. Stress-induced dysbiosis can disrupt immune tolerance and exacerbate autoimmune responses.
-
Neurodegenerative Diseases: Chronic inflammation plays a role in the pathogenesis of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. Stress-induced dysbiosis can promote inflammation in the brain, contributing to neuronal damage and cognitive decline.
Therapeutic Strategies
Targeting the GBA to modulate chronic inflammation is a promising therapeutic strategy for preventing and treating chronic diseases. Potential interventions include:
-
Probiotics: Live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. Probiotics can help restore gut microbial balance, improve gut barrier function, and reduce inflammation.
-
Prebiotics: Non-digestible food ingredients that promote the growth and activity of beneficial bacteria in the gut. Prebiotics can increase the production of SCFAs and other beneficial metabolites.
-
Dietary Interventions: A diet rich in fiber, fruits, vegetables, and whole grains can promote a healthy gut microbiota and reduce inflammation. Avoiding processed foods, sugar, and unhealthy fats is also important.
-
Stress Management Techniques: Techniques such as meditation, yoga, and deep breathing can reduce stress and its impact on the gut microbiota and inflammation.
-
Fecal Microbiota Transplantation (FMT): The transfer of fecal material from a healthy donor to a recipient. FMT can restore gut microbial diversity and function, and it has shown promise in treating certain gastrointestinal disorders and other conditions.
Conclusion
The gut-brain axis plays a critical role in modulating chronic inflammation, a key driver in the progression of many chronic diseases. Stress can disrupt the GBA, leading to dysbiosis, increased intestinal permeability, and systemic inflammation. Targeting the GBA through dietary interventions, probiotics, prebiotics, stress management techniques, and other strategies may offer a promising approach to preventing and treating chronic diseases. Further research is needed to fully understand the complex interactions between the GBA, stress, and chronic inflammation, and to develop targeted therapies that can improve health outcomes.







Leave a Reply