“Leukemia Immunophenotyping: Clinical Applications
Related Articles Leukemia Immunophenotyping: Clinical Applications
- Long-Term Effects Of Chronic Illness On Children – Part 7: Navigating The Labyrinth Of Educational Challenges
- Preventive Screening Guidelines For Chronic Conditions – Part 7
- Social Support Networks For Chronic Disease Patients – Part 10
- Integrative Care Models For Complex Chronic Diseases – Part 6: The Role Of Technology And Telehealth
- The Impact Of Chronic Illness On Mental Health – Part 9
Introduction
With great enthusiasm, let’s explore interesting topics related to Leukemia Immunophenotyping: Clinical Applications. Let’s knit interesting information and provide new insights to readers.
Table of Content
Leukemia Immunophenotyping: Clinical Applications
Introduction
Leukemia, a malignant disorder of the hematopoietic system, is characterized by the uncontrolled proliferation of abnormal blood cells in the bone marrow and peripheral blood. Accurate diagnosis, classification, and risk stratification are essential for effective treatment planning and improved patient outcomes. Immunophenotyping, a powerful flow cytometry-based technique, has emerged as a cornerstone in the diagnostic and prognostic evaluation of leukemia. This article explores the clinical applications of leukemia immunophenotyping, highlighting its role in diagnosis, classification, minimal residual disease (MRD) monitoring, and targeted therapy.
Principles of Immunophenotyping
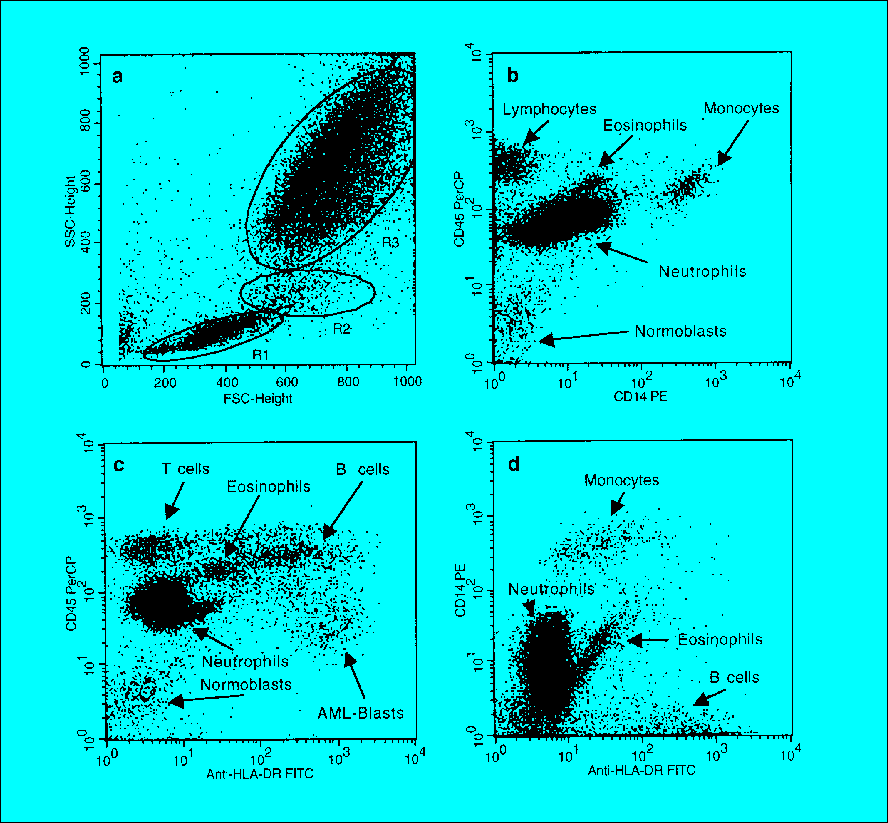
Immunophenotyping relies on the use of fluorescently labeled antibodies that bind to specific cell surface or intracellular antigens (proteins). These antigens, also known as cluster of differentiation (CD) markers, are expressed in a characteristic pattern by different cell types at various stages of differentiation. Flow cytometry allows for the simultaneous detection and quantification of multiple antigens on individual cells within a heterogeneous cell population.
In leukemia immunophenotyping, bone marrow aspirate or peripheral blood samples are stained with a panel of antibodies targeting various CD markers associated with different lineages and stages of hematopoietic development. The resulting data is analyzed using specialized software to identify abnormal cell populations based on their unique immunophenotypic profiles.
Clinical Applications
1. Diagnosis and Classification of Leukemia
Immunophenotyping plays a critical role in the initial diagnosis and classification of leukemia, enabling the differentiation between acute and chronic leukemia, as well as the identification of specific subtypes within each category.
- Acute Leukemia: Acute leukemia is characterized by the rapid proliferation of immature blast cells in the bone marrow. Immunophenotyping is essential for distinguishing between acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), as these two entities require different treatment approaches.
- AML: AML is characterized by the presence of myeloid blasts that express markers such as CD13, CD33, CD117, and myeloperoxidase (MPO). Immunophenotyping can further subclassify AML based on the expression of specific markers associated with different genetic mutations and prognostic risk.
- ALL: ALL is characterized by the presence of lymphoid blasts that express markers such as CD19, CD10, CD34, and terminal deoxynucleotidyl transferase (TdT). Immunophenotyping can distinguish between B-cell ALL (B-ALL) and T-cell ALL (T-ALL), as well as identify specific subtypes with distinct clinical and prognostic features.
- Chronic Leukemia: Chronic leukemia is characterized by the slow accumulation of mature or relatively mature abnormal blood cells. Immunophenotyping is essential for distinguishing between chronic lymphocytic leukemia (CLL) and chronic myeloid leukemia (CML), as well as for monitoring disease progression and response to therapy.
- CLL: CLL is characterized by the presence of clonal B lymphocytes that express CD5, CD19, CD23, and low levels of surface immunoglobulin. Immunophenotyping can identify specific subtypes of CLL with different prognostic implications.
- CML: CML is characterized by the presence of the Philadelphia chromosome (BCR-ABL1 fusion gene), which leads to the uncontrolled proliferation of myeloid cells. Immunophenotyping can detect the presence of abnormal myeloid cells with characteristic immunophenotypic features.
2. Minimal Residual Disease (MRD) Monitoring
MRD refers to the presence of a small number of leukemia cells that remain in the body after treatment. MRD monitoring is essential for assessing the effectiveness of therapy, predicting relapse risk, and guiding treatment decisions.
Immunophenotyping is a sensitive and specific method for MRD detection in both AML and ALL. By using highly sensitive flow cytometry techniques, it is possible to detect leukemia cells at a level of 1 in 10,000 or even 1 in 100,000 normal cells.
- AML MRD Monitoring: In AML, MRD monitoring is typically performed after induction chemotherapy and/or hematopoietic stem cell transplantation (HSCT). The presence of MRD is associated with a higher risk of relapse and poorer overall survival. Patients with persistent MRD may benefit from additional therapy, such as consolidation chemotherapy or donor lymphocyte infusion.
- ALL MRD Monitoring: In ALL, MRD monitoring is performed at multiple time points during treatment, including after induction chemotherapy, consolidation therapy, and maintenance therapy. The level of MRD is a strong predictor of relapse risk. Patients with high levels of MRD may require more intensive therapy, such as HSCT.
3. Targeted Therapy
Immunophenotyping plays a crucial role in identifying patients who are eligible for targeted therapy. Targeted therapies are drugs that specifically target molecules or pathways that are essential for the growth and survival of leukemia cells.
- CD30-Directed Therapy: CD30 is a cell surface marker that is expressed on Hodgkin lymphoma cells and some types of T-cell lymphoma. Brentuximab vedotin is an antibody-drug conjugate that targets CD30 and delivers a cytotoxic agent directly to the cancer cells. Immunophenotyping can be used to identify patients with CD30-positive lymphomas who are likely to respond to brentuximab vedotin.
- CD33-Directed Therapy: CD33 is a cell surface marker that is expressed on myeloid cells. Gemtuzumab ozogamicin is an antibody-drug conjugate that targets CD33 and delivers a cytotoxic agent directly to the cancer cells. Immunophenotyping can be used to identify patients with CD33-positive AML who are likely to respond to gemtuzumab ozogamicin.
- CD20-Directed Therapy: CD20 is a cell surface marker that is expressed on B lymphocytes. Rituximab is a monoclonal antibody that targets CD20 and triggers the destruction of B cells. Immunophenotyping can be used to identify patients with CD20-positive B-cell lymphomas and CLL who are likely to respond to rituximab.
- Blinatumomab: Blinatumomab is a bispecific T-cell engager (BiTE) antibody that binds to CD19 on B cells and CD3 on T cells. This brings the T cells into close proximity with the B cells, allowing the T cells to kill the B cells. Immunophenotyping is used to confirm CD19 expression on the leukemic blasts.
4. Prognostic Stratification
Immunophenotyping can provide valuable prognostic information that can help guide treatment decisions. Certain immunophenotypic features are associated with a higher risk of relapse or treatment failure.
- AML: In AML, immunophenotypic features such as the expression of CD34, CD56, and multidrug resistance proteins are associated with a poorer prognosis.
- ALL: In ALL, immunophenotypic features such as the expression of CD10, CD34, and TdT are associated with a better prognosis.
5. Detection of Aberrant Antigen Expression
Immunophenotyping can identify aberrant antigen expression patterns on leukemia cells. Aberrant antigen expression refers to the expression of antigens that are not normally expressed on cells of that lineage or stage of development. Aberrant antigen expression can be a useful diagnostic marker for leukemia.
- Myeloid Antigen Expression on Lymphoid Blasts: In some cases of ALL, the lymphoid blasts may express myeloid antigens such as CD13 or CD33. This is known as myeloid antigen expression on lymphoid blasts (MAL). MAL is associated with a poorer prognosis in ALL.
- Lymphoid Antigen Expression on Myeloid Blasts: In some cases of AML, the myeloid blasts may express lymphoid antigens such as CD19 or CD7. This is known as lymphoid antigen expression on myeloid blasts (LAL). LAL is associated with a poorer prognosis in AML.
Advantages of Immunophenotyping
- High Sensitivity: Immunophenotyping can detect rare leukemia cells at a level of 1 in 10,000 or even 1 in 100,000 normal cells.
- High Specificity: Immunophenotyping can distinguish between different types of leukemia and identify specific subtypes with distinct clinical and prognostic features.
- Rapid Turnaround Time: Immunophenotyping can be performed quickly, typically within 24-48 hours.
- Relatively Low Cost: Immunophenotyping is a relatively inexpensive test compared to other diagnostic methods, such as cytogenetic analysis and molecular testing.
Limitations of Immunophenotyping
- Subjectivity: The interpretation of immunophenotyping data can be subjective, and different laboratories may have different criteria for defining abnormal cell populations.
- Technical Variability: Immunophenotyping results can be affected by technical factors such as antibody quality, instrument calibration, and sample preparation.
- Limited Information on Genetic Mutations: Immunophenotyping does not provide information on genetic mutations, which are important for risk stratification and targeted therapy.
Conclusion
Immunophenotyping is an indispensable tool in the diagnosis, classification, MRD monitoring, and targeted therapy of leukemia. Its ability to identify and characterize abnormal cell populations based on their unique immunophenotypic profiles has revolutionized the management of leukemia patients. As technology advances and new antibodies are developed, the role of immunophenotyping in leukemia diagnosis and treatment will continue to expand, leading to improved outcomes for patients with this challenging disease.








Leave a Reply