“Genetic Markers in Leukemia Diagnosis: A Comprehensive Overview
Related Articles Genetic Markers in Leukemia Diagnosis: A Comprehensive Overview
- Social Support Networks For Chronic Disease Patients – Part 2
- The Role Of Genetics In Chronic Disease Development – Part 3
- Alternative Therapies For Chronic Pain Management – Part 6
- Chronic Disease Management In Low-Income Communities – Part 8
- Exercise And Physical Activity Guidelines For Chronic Illness Management
Introduction
On this special occasion, we are happy to review interesting topics related to Genetic Markers in Leukemia Diagnosis: A Comprehensive Overview. Come on knit interesting information and provide new insights to readers.
Table of Content
Genetic Markers in Leukemia Diagnosis: A Comprehensive Overview
Leukemia, a heterogeneous group of hematological malignancies, is characterized by the uncontrolled proliferation of abnormal blood cells in the bone marrow. Accurate diagnosis and classification of leukemia are crucial for determining prognosis, treatment strategies, and monitoring disease progression. In recent years, the identification and utilization of genetic markers have revolutionized leukemia diagnosis, providing valuable insights into the underlying molecular mechanisms, improving risk stratification, and enabling the development of targeted therapies. This article provides a comprehensive overview of the role of genetic markers in leukemia diagnosis, covering various types of genetic abnormalities, their diagnostic and prognostic significance, and the evolving landscape of genetic testing technologies.
Understanding Leukemia and Its Subtypes
Leukemia is broadly classified into acute and chronic forms, based on the rate of disease progression. Acute leukemias, such as acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), are characterized by the rapid accumulation of immature blood cells, while chronic leukemias, such as chronic myeloid leukemia (CML) and chronic lymphocytic leukemia (CLL), involve the slower proliferation of more mature cells. Each type of leukemia is further subdivided into various subtypes based on morphology, immunophenotype, and genetic abnormalities.
The Significance of Genetic Markers in Leukemia
Genetic markers play a pivotal role in leukemia diagnosis by:
- Confirming Diagnosis: Genetic markers can help confirm the diagnosis of leukemia, especially in cases with ambiguous morphological or immunophenotypic features.
- Subtyping Leukemia: Genetic abnormalities are often specific to certain leukemia subtypes, allowing for accurate classification and risk stratification.
- Predicting Prognosis: Certain genetic markers are associated with favorable or unfavorable outcomes, providing valuable prognostic information.
- Guiding Treatment Decisions: The presence or absence of specific genetic mutations can influence treatment selection, including chemotherapy regimens, targeted therapies, and stem cell transplantation.
- Monitoring Minimal Residual Disease (MRD): Genetic markers can be used to detect MRD, which is the presence of residual leukemia cells after treatment, helping to predict relapse risk and guide post-treatment management.
Types of Genetic Markers in Leukemia
Genetic markers in leukemia encompass a wide range of abnormalities, including:
-
Chromosomal Translocations:
- Chromosomal translocations involve the exchange of genetic material between two chromosomes.
- Specific translocations are frequently observed in certain leukemia subtypes and have significant diagnostic and prognostic implications.
- Examples of common translocations in leukemia include:
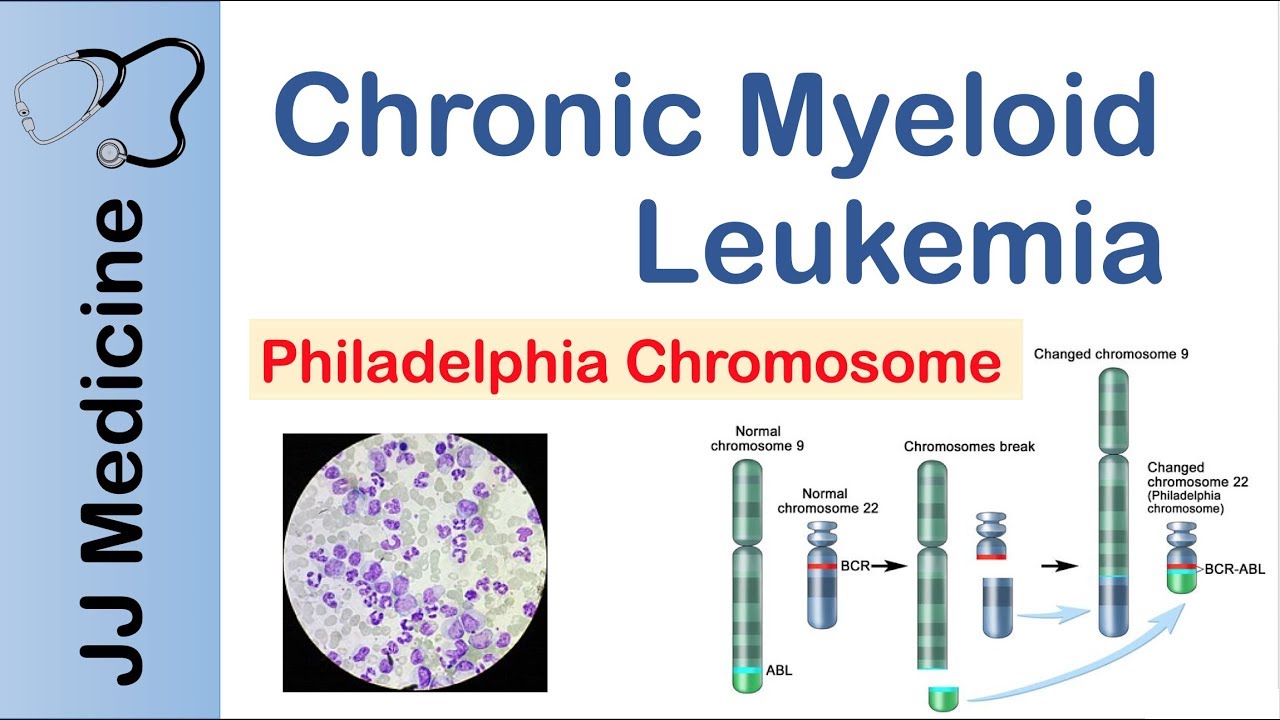
- t(9;22)(q34;q11): Philadelphia chromosome, found in CML and some cases of ALL, resulting in the BCR-ABL1 fusion gene.
- t(15;17)(q22;q12): Found in acute promyelocytic leukemia (APL), leading to the PML-RARA fusion gene.
- t(8;21)(q22;q22): Common in AML, resulting in the RUNX1-RUNX1T1 fusion gene.
- t(11q23): Involving the MLL gene, seen in various leukemia subtypes, particularly in infants and therapy-related leukemias.
-
Gene Mutations:
- Gene mutations involve alterations in the DNA sequence of specific genes.
- Mutations can be point mutations, insertions, deletions, or duplications.
- Several genes are frequently mutated in leukemia, including:
- FLT3: A receptor tyrosine kinase, commonly mutated in AML, associated with poor prognosis.
- NPM1: A nucleolar protein, frequently mutated in AML, often associated with favorable prognosis.
- IDH1/IDH2: Isocitrate dehydrogenase genes, mutated in AML, affecting DNA methylation and cell differentiation.
- TP53: A tumor suppressor gene, mutated in various leukemia subtypes, associated with poor prognosis.
- RUNX1: A transcription factor, mutated in AML and ALL, affecting hematopoietic development.
-
Copy Number Alterations (CNAs):
- CNAs involve gains or losses of large segments of DNA, leading to changes in gene dosage.
- CNAs can be detected using techniques such as array-based comparative genomic hybridization (aCGH) or single nucleotide polymorphism (SNP) arrays.
- Common CNAs in leukemia include:
- Deletions of tumor suppressor genes, such as TP53, RB1, or CDKN2A.
- Amplifications of oncogenes, such as MYC or ERG.
-
Epigenetic Modifications:
- Epigenetic modifications involve changes in gene expression without altering the DNA sequence.
- Examples of epigenetic modifications include DNA methylation and histone modifications.
- Epigenetic alterations can play a role in leukemia development and progression.
- Mutations in genes involved in epigenetic regulation, such as DNMT3A, TET2, and ASXL1, are frequently observed in AML.
Genetic Testing Technologies for Leukemia Diagnosis
Several genetic testing technologies are used in leukemia diagnosis, including:
-
Cytogenetics:
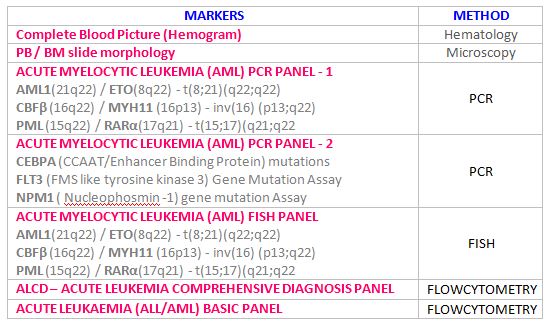
- Cytogenetics involves the analysis of chromosomes using techniques such as karyotyping and fluorescence in situ hybridization (FISH).
- Karyotyping allows for the visualization of the entire chromosome complement, detecting numerical and structural abnormalities.
- FISH uses fluorescent probes to detect specific DNA sequences, allowing for the identification of translocations, deletions, and amplifications.
-
Polymerase Chain Reaction (PCR):
- PCR is a molecular biology technique used to amplify specific DNA sequences.
- Real-time PCR (qPCR) allows for the quantification of DNA or RNA, enabling the detection of minimal residual disease (MRD).
- Reverse transcription PCR (RT-PCR) is used to detect RNA transcripts, such as fusion genes.
-
Next-Generation Sequencing (NGS):
- NGS is a high-throughput sequencing technology that allows for the simultaneous analysis of multiple genes or the entire genome.
- NGS can detect a wide range of genetic abnormalities, including mutations, CNAs, and gene fusions.
- NGS is increasingly used in leukemia diagnosis for comprehensive genomic profiling and personalized medicine.
-
Microarray Analysis:
- Microarray analysis is a technique used to measure the expression levels of thousands of genes simultaneously.
- Microarrays can be used to identify gene expression signatures associated with specific leukemia subtypes or prognostic groups.
- SNP arrays can be used to detect CNAs and loss of heterozygosity (LOH).
Clinical Applications of Genetic Markers in Leukemia Diagnosis
Genetic markers have numerous clinical applications in leukemia diagnosis, including:
-
Diagnosis and Classification:
- Genetic markers can confirm the diagnosis of leukemia and classify it into specific subtypes.
- The presence of certain genetic abnormalities, such as the Philadelphia chromosome in CML or the PML-RARA fusion gene in APL, is diagnostic for these specific subtypes.
-
Risk Stratification:
- Genetic markers can be used to stratify patients into different risk groups based on their likelihood of response to treatment and overall survival.
- For example, in AML, patients with FLT3-ITD mutations are considered to be at higher risk, while those with NPM1 mutations are often associated with favorable outcomes.
-
Treatment Selection:
- Genetic markers can influence treatment selection, including chemotherapy regimens, targeted therapies, and stem cell transplantation.
- For example, patients with the Philadelphia chromosome in CML are treated with tyrosine kinase inhibitors (TKIs) that specifically target the BCR-ABL1 fusion protein.
- Patients with FLT3-ITD mutations in AML may benefit from treatment with FLT3 inhibitors.
-
Monitoring Minimal Residual Disease (MRD):
- Genetic markers can be used to detect MRD, which is the presence of residual leukemia cells after treatment.
- MRD detection can help predict relapse risk and guide post-treatment management.
- Techniques such as qPCR and flow cytometry are used to monitor MRD using specific genetic markers.
-
Targeted Therapy Development:
- The identification of specific genetic mutations in leukemia has led to the development of targeted therapies that specifically inhibit the function of the mutated protein.
- Examples of targeted therapies in leukemia include TKIs for CML, FLT3 inhibitors for AML, and IDH inhibitors for AML.
Conclusion
Genetic markers have become indispensable tools in leukemia diagnosis, providing valuable information for accurate classification, risk stratification, treatment selection, and monitoring of minimal residual disease. The continued advancement of genetic testing technologies, such as NGS, has expanded our understanding of the molecular landscape of leukemia and enabled the development of personalized treatment strategies. As our knowledge of leukemia genetics continues to grow, genetic markers will play an increasingly important role in improving patient outcomes and advancing the field of leukemia research.







Leave a Reply