“Comparative Analysis of Leukemia Subtypes
Related Articles Comparative Analysis of Leukemia Subtypes
- The Role Of Stress In Chronic Disease Progression – Part 9: The Gut-Brain Axis And The Modulation Of Chronic Inflammation
- Innovations In Medical Devices For Chronic Disease Management – Part 10: The Rise Of Personalized And Predictive Health Technologies
- Workplace Accommodations For Employees With Chronic Diseases – Part 3
- Dietary Strategies For Coping With Chronic Diseases – Part 9
- Social Determinants Of Health And Chronic Disease Outcomes – Part 3
Introduction
With great enthusiasm, let’s explore interesting topics related to Comparative Analysis of Leukemia Subtypes. Come on knit interesting information and provide new insights to readers.
Table of Content
Comparative Analysis of Leukemia Subtypes
Leukemia, a malignant neoplasm of the hematopoietic system, is characterized by the uncontrolled proliferation of abnormal leukocytes in the bone marrow and peripheral blood. This heterogeneous group of disorders is classified into various subtypes based on the cell lineage involved (myeloid or lymphoid), the maturity of the leukemic cells (acute or chronic), and specific genetic and molecular abnormalities. Understanding the distinctions between these subtypes is crucial for accurate diagnosis, risk stratification, and personalized treatment strategies. This article provides a comparative analysis of the major leukemia subtypes, highlighting their unique characteristics, diagnostic features, prognostic factors, and therapeutic approaches.
Classification of Leukemia Subtypes
Leukemias are broadly classified into four main types: acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphocytic leukemia (CLL). Acute leukemias are characterized by the rapid proliferation of immature blast cells, while chronic leukemias involve the accumulation of more mature, but still abnormal, leukocytes.
Acute Myeloid Leukemia (AML)
AML is a heterogeneous group of disorders characterized by the clonal expansion of myeloid blasts in the bone marrow, peripheral blood, and other tissues. It accounts for the majority of acute leukemias in adults.
-
Etiology and Risk Factors: The etiology of AML is multifactorial, involving both genetic and environmental factors. Risk factors include exposure to ionizing radiation, certain chemicals (e.g., benzene), prior chemotherapy or radiation therapy, and inherited genetic syndromes (e.g., Down syndrome, Fanconi anemia).
-
Pathophysiology: AML arises from mutations in hematopoietic stem cells or early progenitor cells, leading to impaired differentiation and uncontrolled proliferation. These mutations often involve genes that regulate transcription, cell signaling, and epigenetic modification.
-
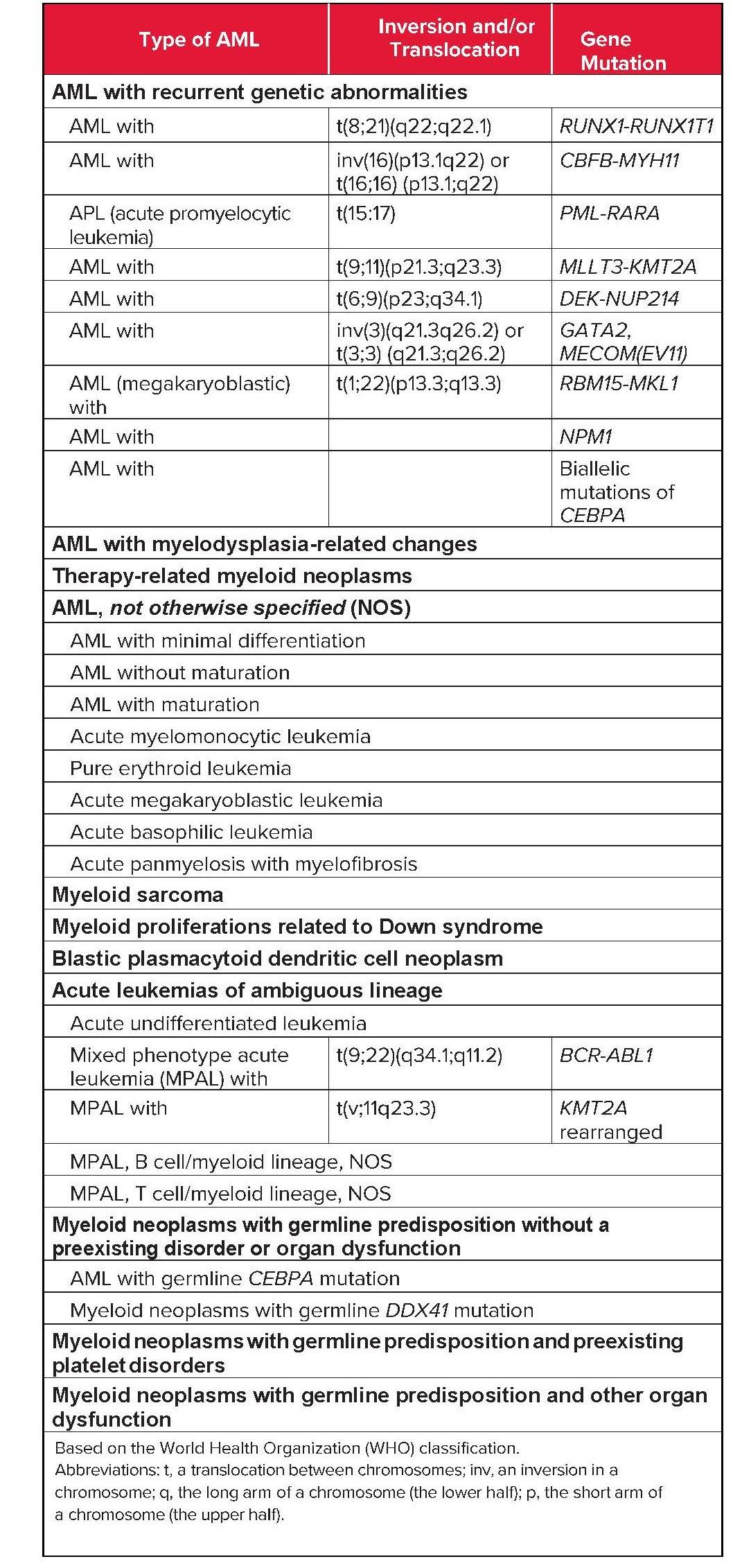
Subtypes: AML is further classified into several subtypes based on the World Health Organization (WHO) classification, which incorporates morphology, immunophenotype, cytogenetics, and molecular abnormalities. Some common subtypes include:
- AML with recurrent genetic abnormalities: This category includes AML with specific translocations or inversions, such as t(8;21), t(15;17), inv(16), and t(9;11), which are associated with distinct clinical features and prognosis.
- AML with myelodysplasia-related changes: This subtype arises from pre-existing myelodysplastic syndromes (MDS) or de novo with MDS-related cytogenetic abnormalities.
- Therapy-related AML: This subtype develops as a late complication of prior chemotherapy or radiation therapy for other malignancies.
- AML not otherwise specified (NOS): This category includes AML cases that do not fit into the other defined subtypes.
-
Clinical Features: Patients with AML typically present with symptoms related to bone marrow failure, such as fatigue, pallor, bleeding, and infections. Other symptoms may include bone pain, lymphadenopathy, splenomegaly, and skin infiltration.
-
Diagnosis: The diagnosis of AML requires bone marrow aspiration and biopsy, which demonstrate an increased number of myeloid blasts (≥20%) and characteristic morphologic features. Immunophenotyping by flow cytometry is essential for identifying the lineage of the leukemic cells and detecting specific surface markers. Cytogenetic analysis and molecular testing are crucial for identifying genetic abnormalities that guide risk stratification and treatment decisions.
-
Prognosis: The prognosis of AML varies widely depending on several factors, including age, performance status, cytogenetic and molecular abnormalities, and response to therapy. Favorable-risk AML is associated with specific genetic mutations (e.g., NPM1 mutation without FLT3-ITD) and is more likely to achieve remission with standard chemotherapy. Adverse-risk AML is characterized by unfavorable cytogenetic abnormalities (e.g., complex karyotype, monosomy 7) or mutations (e.g., FLT3-ITD, TP53 mutation) and is associated with a higher risk of relapse and shorter survival.
-
Treatment: The treatment of AML typically involves induction chemotherapy followed by consolidation therapy. Induction chemotherapy aims to eradicate the leukemic blasts from the bone marrow and achieve complete remission. Consolidation therapy is administered to eliminate any residual leukemic cells and prevent relapse. Hematopoietic stem cell transplantation (HSCT) is often considered for patients with high-risk AML or those who relapse after initial therapy. Targeted therapies, such as FLT3 inhibitors (e.g., midostaurin, gilteritinib) and IDH inhibitors (e.g., enasidenib, ivosidenib), have shown promising results in patients with specific genetic mutations.
Acute Lymphoblastic Leukemia (ALL)
ALL is a malignancy of lymphoid progenitor cells, primarily affecting B-cell or T-cell lineages. It is the most common type of leukemia in children but can also occur in adults.
-
Etiology and Risk Factors: The etiology of ALL is not fully understood, but genetic factors, environmental exposures, and immune dysfunction may play a role. Risk factors include exposure to ionizing radiation, certain chemicals (e.g., benzene), and inherited genetic syndromes (e.g., Down syndrome, neurofibromatosis).
-
Pathophysiology: ALL arises from mutations in lymphoid progenitor cells, leading to impaired differentiation and uncontrolled proliferation. These mutations often involve genes that regulate lymphocyte development, cell signaling, and cell cycle control.
-
Subtypes: ALL is classified into B-cell ALL (B-ALL) and T-cell ALL (T-ALL) based on the lineage of the leukemic cells. B-ALL is further subdivided into several subtypes based on cytogenetic and molecular abnormalities, such as:
- B-ALL with t(9;22)(q34;q11.2) (Philadelphia chromosome-positive ALL): This subtype is characterized by the presence of the BCR-ABL1 fusion gene and is associated with a poorer prognosis.
- B-ALL with t(v;11q23) (MLL-rearranged ALL): This subtype is characterized by rearrangements of the MLL gene and is more common in infants.
- B-ALL with t(12;21)(p13;q22) (ETV6-RUNX1 ALL): This subtype is associated with a favorable prognosis, particularly in children.
- B-ALL with hyperdiploidy: This subtype is characterized by the presence of more than 50 chromosomes and is also associated with a favorable prognosis.
-
Clinical Features: Patients with ALL typically present with symptoms related to bone marrow failure, such as fatigue, pallor, bleeding, and infections. Other symptoms may include bone pain, lymphadenopathy, splenomegaly, and mediastinal mass (in T-ALL).
-
Diagnosis: The diagnosis of ALL requires bone marrow aspiration and biopsy, which demonstrate an increased number of lymphoblasts (≥25%) and characteristic morphologic features. Immunophenotyping by flow cytometry is essential for identifying the lineage of the leukemic cells and detecting specific surface markers. Cytogenetic analysis and molecular testing are crucial for identifying genetic abnormalities that guide risk stratification and treatment decisions.
-
Prognosis: The prognosis of ALL varies depending on several factors, including age, white blood cell count at diagnosis, cytogenetic and molecular abnormalities, and response to therapy. Children with ALL generally have a better prognosis than adults. Favorable-risk ALL is associated with specific genetic abnormalities (e.g., ETV6-RUNX1 fusion, hyperdiploidy) and is more likely to achieve long-term remission with intensive chemotherapy. Adverse-risk ALL is characterized by unfavorable cytogenetic abnormalities (e.g., Philadelphia chromosome, MLL rearrangement) or mutations (e.g., TP53 mutation) and is associated with a higher risk of relapse and shorter survival.
-
Treatment: The treatment of ALL typically involves intensive chemotherapy, including induction, consolidation, and maintenance phases. Induction chemotherapy aims to eradicate the leukemic blasts from the bone marrow and achieve complete remission. Consolidation therapy is administered to eliminate any residual leukemic cells and prevent relapse. Maintenance therapy is a prolonged course of low-dose chemotherapy that helps to maintain remission. Central nervous system (CNS) prophylaxis is an important component of ALL treatment to prevent leukemic cells from infiltrating the brain and spinal cord. HSCT is often considered for patients with high-risk ALL or those who relapse after initial therapy. Targeted therapies, such as tyrosine kinase inhibitors (e.g., imatinib, dasatinib, ponatinib) for Philadelphia chromosome-positive ALL and monoclonal antibodies (e.g., blinatumomab, inotuzumab ozogamicin) for relapsed/refractory ALL, have significantly improved outcomes.
Chronic Myeloid Leukemia (CML)
CML is a myeloproliferative neoplasm characterized by the presence of the Philadelphia chromosome, which results from a reciprocal translocation between chromosomes 9 and 22 [t(9;22)(q34;q11.2)]. This translocation leads to the formation of the BCR-ABL1 fusion gene, which encodes a constitutively active tyrosine kinase that drives the uncontrolled proliferation of myeloid cells.
-
Etiology and Risk Factors: The etiology of CML is not fully understood, but exposure to ionizing radiation is a known risk factor.
-
Pathophysiology: The BCR-ABL1 fusion gene is the primary driver of CML. The BCR-ABL1 tyrosine kinase phosphorylates various downstream targets, leading to increased cell proliferation, decreased apoptosis, and impaired differentiation of myeloid cells.
-
Phases: CML typically progresses through three phases: chronic phase, accelerated phase, and blast crisis. The chronic phase is characterized by a relatively stable white blood cell count and minimal symptoms. The accelerated phase is marked by an increasing white blood cell count, resistance to therapy, and the appearance of additional cytogenetic abnormalities. Blast crisis is characterized by the accumulation of immature blast cells in the bone marrow and peripheral blood, resembling acute leukemia.
-
Clinical Features: Patients with CML may be asymptomatic in the early stages of the disease. As the disease progresses, symptoms may include fatigue, weight loss, night sweats, splenomegaly, and abdominal discomfort.
-
Diagnosis: The diagnosis of CML is based on the presence of the Philadelphia chromosome or the BCR-ABL1 fusion gene in peripheral blood or bone marrow cells. A complete blood count typically shows an elevated white blood cell count with a left shift (increased immature granulocytes). Bone marrow aspiration and biopsy may be performed to assess the percentage of blast cells and evaluate for disease progression.
-
Prognosis: The introduction of tyrosine kinase inhibitors (TKIs) has dramatically improved the prognosis of CML. TKIs, such as imatinib, dasatinib, nilotinib, and bosutinib, specifically target the BCR-ABL1 tyrosine kinase and effectively control the disease in most patients. Patients who achieve a deep molecular response (undetectable BCR-ABL1 transcript levels) may be able to discontinue TKI therapy under close monitoring.
-
Treatment: The primary treatment for CML is TKI therapy. HSCT is reserved for patients who fail to respond to TKI therapy or progress to accelerated phase or blast crisis.
Chronic Lymphocytic Leukemia (CLL)
CLL is a malignancy of mature B lymphocytes characterized by the accumulation of clonal B cells in the bone marrow, peripheral blood, and lymphoid tissues.
-
Etiology and Risk Factors: The etiology of CLL is not fully understood, but genetic factors and immune dysfunction may play a role.
-
Pathophysiology: CLL cells are characterized by defects in apoptosis and increased survival. These cells accumulate in the bone marrow, peripheral blood, and lymphoid tissues, leading to lymphadenopathy, splenomegaly, and bone marrow failure.
-
Clinical Features: Many patients with CLL are asymptomatic at diagnosis and are identified during routine blood tests. As the disease progresses, symptoms may include fatigue, lymphadenopathy, splenomegaly, recurrent infections, and autoimmune cytopenias.
-
Diagnosis: The diagnosis of CLL is based on the presence of at least 5,000 clonal B lymphocytes per microliter of peripheral blood for at least 3 months. Immunophenotyping by flow cytometry is essential for confirming the diagnosis and identifying characteristic surface markers, such as CD5, CD19, and CD23. Bone marrow aspiration and biopsy may be performed to assess the extent of bone marrow involvement. Cytogenetic analysis and molecular testing are important for risk stratification.
-
Prognosis: The prognosis of CLL varies depending on several factors, including disease stage, genetic abnormalities, and response to therapy. The Rai and Binet staging systems are commonly used to assess disease burden and predict prognosis. Unfavorable prognostic factors include del(17p), TP53 mutation, unmutated IGHV genes, and high levels of beta-2 microglobulin.
-
Treatment: The treatment of CLL depends on the stage of the disease, the presence of symptoms, and the patient’s overall health. Asymptomatic patients with early-stage CLL may be monitored without treatment ("watch and wait" approach). Treatment is typically initiated when patients develop symptoms, such as fatigue, lymphadenopathy, splenomegaly, or cytopenias. Treatment options include chemotherapy (e.g., fludarabine, cyclophosphamide, bendamustine), monoclonal antibodies (e.g., rituximab, obinutuzumab), targeted therapies (e.g., ibrutinib, acalabrutinib, venetoclax), and HSCT.
Conclusion
Leukemia is a complex and heterogeneous group of disorders with diverse subtypes, each characterized by unique clinical features, diagnostic criteria, prognostic factors, and therapeutic approaches. Accurate classification and risk stratification are essential for guiding treatment decisions and improving patient outcomes. Advances in molecular diagnostics and targeted therapies have significantly improved the prognosis of many leukemia subtypes, highlighting the importance of personalized medicine in the management of these malignancies. Further research is needed to develop novel therapies for high-risk leukemia and to improve the long-term outcomes for all patients with leukemia.








Leave a Reply