“Age-Related Changes in Bone Microarchitecture
Related Articles Age-Related Changes in Bone Microarchitecture
- Rheumatoid Arthritis Vs. Osteoarthritis: Key Differences
- The Cornerstone Of Strong Bones: Unveiling The Vital Role Of Calcium
- The Role Of Hormones In Bone Growth And Development
- The Impact Of Hormonal Changes On Bone Health
- Bone Health And Chronic Kidney Disease
Introduction
With great enthusiasm, let’s explore interesting topics related to Age-Related Changes in Bone Microarchitecture. Let’s knit interesting information and provide new insights to readers.
Age-Related Changes in Bone Microarchitecture
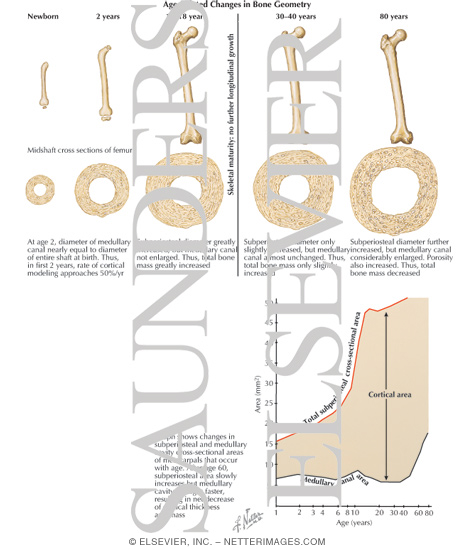
Introduction
Bone is a dynamic tissue that undergoes continuous remodeling throughout life. This remodeling process involves the coordinated action of bone-resorbing osteoclasts and bone-forming osteoblasts, ensuring bone maintenance, repair, and adaptation to mechanical demands. However, with advancing age, the delicate balance between bone resorption and formation is disrupted, leading to a gradual decline in bone mass and deterioration of bone microarchitecture. These age-related changes in bone microarchitecture significantly contribute to increased bone fragility and a higher risk of fractures, particularly in older adults.
Bone Microarchitecture: A Key Determinant of Bone Strength
Bone strength is determined not only by bone mineral density (BMD) but also by bone microarchitecture, which refers to the intricate three-dimensional arrangement of bone tissue. Bone microarchitecture plays a crucial role in determining bone’s resistance to fracture. It encompasses various structural parameters, including trabecular thickness, trabecular separation, trabecular number, cortical thickness, and cortical porosity.
- Trabecular Bone: Trabecular bone, also known as cancellous bone, is a porous network of interconnected plates and rods found in the interior of bones, particularly in the vertebrae, femoral neck, and distal radius. Trabecular architecture provides structural support and contributes significantly to bone’s ability to withstand compressive forces.
- Cortical Bone: Cortical bone, also known as compact bone, is the dense outer layer of bone that provides strength and protection. Cortical thickness and porosity are important determinants of bone strength, particularly in long bones.
Age-Related Changes in Bone Microarchitecture
As individuals age, bone microarchitecture undergoes significant changes that compromise bone strength and increase fracture risk. These changes include:
- Trabecular Thinning and Loss: One of the most prominent age-related changes in bone microarchitecture is the thinning and loss of trabeculae. This process occurs due to an imbalance between bone resorption and formation, with increased osteoclast activity and decreased osteoblast activity. As trabeculae become thinner and fewer in number, the structural integrity of trabecular bone is compromised, leading to reduced bone strength and increased susceptibility to fractures.
- Trabecular Disconnection: In addition to thinning and loss, trabeculae can also become disconnected, further disrupting the structural network of trabecular bone. Trabecular disconnection reduces the load-bearing capacity of bone and increases its vulnerability to fracture.
- Cortical Thinning: Cortical bone also undergoes age-related changes, including cortical thinning. This thinning occurs due to increased endocortical resorption, where bone is resorbed from the inner surface of the cortex. Cortical thinning reduces the overall strength and stiffness of bone, making it more prone to fracture.
- Increased Cortical Porosity: Cortical porosity, the presence of small holes or pores within the cortical bone, increases with age. These pores weaken the cortical bone and reduce its resistance to fracture.
- Increased Bone Marrow Adiposity: Bone marrow adiposity, the accumulation of fat cells in the bone marrow, increases with age. Increased bone marrow adiposity is associated with decreased bone formation and increased bone resorption, contributing to the deterioration of bone microarchitecture.
- Reduced Osteocyte Density and Function: Osteocytes, the most abundant cells in bone, play a crucial role in maintaining bone health. With age, osteocyte density and function decline, impairing their ability to sense mechanical loads and regulate bone remodeling. This decline in osteocyte activity contributes to the deterioration of bone microarchitecture.
Mechanisms Underlying Age-Related Changes in Bone Microarchitecture
The age-related changes in bone microarchitecture are driven by a complex interplay of hormonal, genetic, and environmental factors. Some of the key mechanisms include:
- Hormonal Changes: Hormonal changes, particularly the decline in estrogen levels in women after menopause and the decline in testosterone levels in men, play a significant role in age-related bone loss and deterioration of bone microarchitecture. Estrogen and testosterone are essential for maintaining bone mass and promoting bone formation. Their decline leads to increased bone resorption and decreased bone formation, resulting in bone loss and compromised bone microarchitecture.
- Inflammation: Chronic inflammation, which is common in older adults, can contribute to bone loss and deterioration of bone microarchitecture. Inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), stimulate osteoclast activity and inhibit osteoblast activity, leading to increased bone resorption and decreased bone formation.
- Oxidative Stress: Oxidative stress, an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to neutralize them, increases with age. ROS can damage bone cells, including osteoblasts and osteocytes, and impair their function, contributing to bone loss and deterioration of bone microarchitecture.
- Genetic Factors: Genetic factors play a significant role in determining an individual’s peak bone mass and their susceptibility to age-related bone loss. Certain genes are associated with increased bone density and reduced fracture risk, while others are associated with decreased bone density and increased fracture risk.
- Lifestyle Factors: Lifestyle factors, such as diet, physical activity, and smoking, can also influence bone health and bone microarchitecture. A diet deficient in calcium and vitamin D, lack of physical activity, and smoking can all contribute to bone loss and deterioration of bone microarchitecture.
Assessment of Bone Microarchitecture
Several techniques are available to assess bone microarchitecture, including:
- High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT): HR-pQCT is a non-invasive imaging technique that provides detailed three-dimensional images of bone microarchitecture at peripheral skeletal sites, such as the distal radius and tibia. HR-pQCT can measure various structural parameters, including trabecular thickness, trabecular separation, trabecular number, cortical thickness, and cortical porosity.
- Magnetic Resonance Imaging (MRI): MRI can also be used to assess bone microarchitecture, particularly in the spine and hip. MRI can provide information about trabecular bone volume, trabecular thickness, and trabecular connectivity.
- Bone Biopsy: Bone biopsy is an invasive procedure that involves removing a small sample of bone tissue for microscopic examination. Bone biopsy can provide detailed information about bone microarchitecture, including trabecular structure, cortical thickness, and bone cell activity.
Clinical Significance of Age-Related Changes in Bone Microarchitecture
Age-related changes in bone microarchitecture have significant clinical implications, as they contribute to increased bone fragility and a higher risk of fractures. Fractures, particularly hip fractures, are a major cause of morbidity and mortality in older adults. They can lead to pain, disability, loss of independence, and increased healthcare costs.
Strategies to Mitigate Age-Related Changes in Bone Microarchitecture
Several strategies can help mitigate age-related changes in bone microarchitecture and reduce fracture risk:
- Lifestyle Modifications: Lifestyle modifications, such as a diet rich in calcium and vitamin D, regular weight-bearing exercise, and smoking cessation, can help maintain bone health and improve bone microarchitecture.
- Pharmacological Interventions: Several medications are available to treat osteoporosis and reduce fracture risk. These medications include bisphosphonates, denosumab, selective estrogen receptor modulators (SERMs), and teriparatide. These medications can increase bone mineral density and improve bone microarchitecture.
- Fall Prevention Strategies: Fall prevention strategies, such as home safety modifications, balance training, and vision correction, can help reduce the risk of falls and fractures.
Conclusion
Age-related changes in bone microarchitecture are a significant contributor to increased bone fragility and a higher risk of fractures in older adults. These changes include trabecular thinning and loss, trabecular disconnection, cortical thinning, increased cortical porosity, increased bone marrow adiposity, and reduced osteocyte density and function. These changes are driven by a complex interplay of hormonal, genetic, and environmental factors. Assessment of bone microarchitecture can be performed using techniques such as HR-pQCT, MRI, and bone biopsy. Strategies to mitigate age-related changes in bone microarchitecture include lifestyle modifications, pharmacological interventions, and fall prevention strategies. By addressing these changes, we can improve bone health and reduce fracture risk in older adults.








Leave a Reply