“Clinical Trials in Leukemia Treatment: Patient Considerations
Related Articles Clinical Trials in Leukemia Treatment: Patient Considerations
- Environmental Factors And Chronic Disease Risk – Part 3
- Diet And Nutrition Recommendations For Leukemia Patients
- Psychological Resilience In Chronic Disease Patients: Navigating Challenges And Fostering Well-being
- The Role Of Immunotherapy In Leukemia Treatment
- Chronic Disease Trends In Aging Populations – Part 8
Introduction
We will be happy to explore interesting topics related to Clinical Trials in Leukemia Treatment: Patient Considerations. Come on knit interesting information and provide new insights to readers.
Table of Content
Clinical Trials in Leukemia Treatment: Patient Considerations
Leukemia, a cancer of the blood and bone marrow, presents a complex landscape of diagnoses and treatment options. While standard treatments like chemotherapy, radiation therapy, and stem cell transplantation have improved outcomes for many patients, leukemia remains a life-threatening disease. Clinical trials offer hope for patients seeking innovative therapies and the potential for better outcomes. However, participating in a clinical trial is a significant decision that requires careful consideration. This article delves into the critical aspects of clinical trials in leukemia treatment, focusing on patient considerations and providing insights to help individuals make informed choices.
Understanding Clinical Trials
Clinical trials are research studies designed to evaluate the safety and effectiveness of new medical interventions, such as drugs, therapies, or medical devices. These trials are essential for advancing medical knowledge and improving patient care. In leukemia treatment, clinical trials may explore novel targeted therapies, immunotherapies, or combinations of existing treatments.
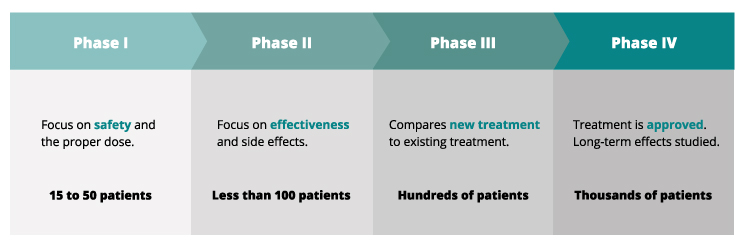
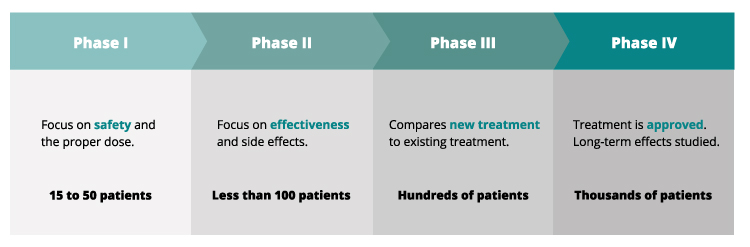
Phases of Clinical Trials
Clinical trials typically progress through several phases, each with a specific purpose:
- Phase 1: These trials primarily focus on safety and determining the appropriate dosage of a new treatment. They usually involve a small number of healthy volunteers or patients with advanced cancer who have exhausted other treatment options.
- Phase 2: Phase 2 trials evaluate the effectiveness of the treatment and further assess its safety in a larger group of patients with leukemia. Researchers monitor the treatment’s impact on the disease and identify potential side effects.
- Phase 3: These trials compare the new treatment to the current standard treatment. They involve a larger number of patients and aim to confirm the treatment’s effectiveness, monitor side effects, and compare it to existing therapies.
- Phase 4: Phase 4 trials are conducted after a treatment has been approved and is available to the public. They gather additional information about the treatment’s long-term effects, optimal use, and potential risks or benefits in specific patient populations.
Benefits of Participating in Clinical Trials
Participating in a clinical trial can offer several potential benefits for patients with leukemia:
- Access to Innovative Treatments: Clinical trials provide access to cutting-edge therapies that may not be available through standard treatment options. These novel treatments may offer the potential for improved outcomes or a better quality of life.
- Potential for Improved Outcomes: Clinical trials aim to improve treatment outcomes for patients with leukemia. By participating, patients may have the opportunity to benefit from more effective therapies or treatment strategies.
- Close Monitoring and Care: Clinical trial participants receive close monitoring and care from a team of healthcare professionals. This may include more frequent check-ups, comprehensive assessments, and personalized support.
- Contribution to Medical Advancement: By participating in a clinical trial, patients contribute to the advancement of medical knowledge and the development of new treatments for leukemia. Their involvement can help improve the lives of future patients.
- Potential for Financial Assistance: Some clinical trials may offer financial assistance to cover expenses related to treatment, travel, or accommodation. This can help alleviate the financial burden of participating in a trial.
Risks of Participating in Clinical Trials
While clinical trials offer potential benefits, it is essential to be aware of the potential risks:
- Unknown Side Effects: New treatments being tested in clinical trials may have unknown side effects. Participants may experience unexpected or severe adverse reactions.
- Treatment May Not Be Effective: There is no guarantee that the treatment being tested in a clinical trial will be effective. Some participants may not experience any benefit from the treatment.
- Time Commitment: Participating in a clinical trial can require a significant time commitment. Patients may need to attend frequent appointments, undergo extensive testing, and adhere to strict protocols.
- Placebo Effect: Some clinical trials involve a placebo group, where participants receive a sham treatment instead of the active treatment. Patients in the placebo group may not experience any benefit.
- Insurance Coverage: Insurance coverage for clinical trial participation can vary. Patients should check with their insurance provider to understand what costs will be covered.
Patient Considerations Before Participating in a Clinical Trial
Before deciding to participate in a clinical trial, patients with leukemia should carefully consider the following factors:
- Understanding the Trial Protocol: Patients should thoroughly understand the trial protocol, including the purpose of the trial, the treatment being tested, the potential benefits and risks, and the required procedures and assessments.
- Discussing with Your Doctor: Patients should discuss the clinical trial with their doctor or oncologist. They can provide valuable insights into the trial’s suitability for the patient’s specific condition and medical history.
- Evaluating Eligibility Criteria: Clinical trials have specific eligibility criteria that patients must meet to participate. These criteria may include factors such as age, disease stage, prior treatments, and overall health status.
- Assessing Personal Circumstances: Patients should consider their personal circumstances, such as their ability to travel to the trial site, their time commitment, and their financial resources.
- Informed Consent: Patients must provide informed consent before participating in a clinical trial. This means they must understand the trial’s purpose, procedures, potential benefits and risks, and their right to withdraw from the trial at any time.
- Asking Questions: Patients should not hesitate to ask questions about any aspect of the clinical trial. They should seek clarification on any points they do not understand.
- Seeking a Second Opinion: Patients may want to seek a second opinion from another doctor or oncologist before making a decision about participating in a clinical trial.
- Reviewing the Consent Form: Patients should carefully review the consent form before signing it. They should ensure that they understand all the information provided and that they agree to the terms of the trial.
- Discussing with Family and Friends: Patients may want to discuss their decision with family and friends. They can provide emotional support and help patients weigh the pros and cons of participating in the trial.
- Considering the Impact on Quality of Life: Patients should consider how participating in the clinical trial may impact their quality of life. They should weigh the potential benefits of the trial against the potential risks and inconveniences.
- Evaluating the Trial’s Scientific Rigor: Patients should evaluate the trial’s scientific rigor by considering factors such as the study design, the sample size, and the data analysis methods.
- Understanding the Trial’s Funding Source: Patients should understand the trial’s funding source, as this may influence the trial’s objectives and outcomes.
- Considering the Trial’s Ethical Aspects: Patients should consider the trial’s ethical aspects, such as the protection of patient privacy and the minimization of risks.
- Weighing the Potential Benefits and Risks: Patients should carefully weigh the potential benefits and risks of participating in the clinical trial. They should consider their personal values and priorities when making their decision.
- Making an Informed Decision: Ultimately, the decision to participate in a clinical trial is a personal one. Patients should make an informed decision based on their understanding of the trial, their personal circumstances, and their values.
Finding Clinical Trials for Leukemia
Patients can find clinical trials for leukemia through various resources:
- Physician Referral: The patient’s doctor or oncologist may be able to recommend clinical trials that are suitable for their specific condition.
- ClinicalTrials.gov: This website, maintained by the National Institutes of Health, provides a comprehensive database of clinical trials worldwide.
- The Leukemia & Lymphoma Society: This organization offers resources and support for patients with leukemia, including information about clinical trials.
- Cancer Research UK: This organization provides information about cancer research and clinical trials in the United Kingdom.
- Patient Advocacy Groups: Many patient advocacy groups focus on specific types of leukemia and provide information about clinical trials.
Conclusion
Clinical trials offer a valuable opportunity for patients with leukemia to access innovative treatments and contribute to the advancement of medical knowledge. However, participating in a clinical trial is a significant decision that requires careful consideration. By understanding the benefits and risks of clinical trials, evaluating their personal circumstances, and discussing the trial with their doctor, patients can make informed choices that align with their values and priorities.
Disclaimer: This article is intended for informational purposes only and does not constitute medical advice. Patients with leukemia should consult with their doctor or oncologist to discuss their treatment options and determine whether a clinical trial is right for them.





Leave a Reply