“Chronic Myeloid Leukemia (CML): Current Treatment Strategies
Related Articles Chronic Myeloid Leukemia (CML): Current Treatment Strategies
- Educational Interventions For Chronic Disease Prevention – Part 10: Leveraging Technology And Digital Health Platforms
- Lifestyle Changes To Manage Chronic Conditions – Part 10
- Innovations In Medical Devices For Chronic Disease Management – Part 2
- The Role Of Genetics In Chronic Disease Development – Part 7
- Long-term Effects Of Chronic Illness On Children – Part 5: Navigating The Educational Landscape
Introduction
With great enthusiasm, let’s explore interesting topics related to Chronic Myeloid Leukemia (CML): Current Treatment Strategies. Let’s knit interesting information and provide new insights to readers.
Table of Content
Chronic Myeloid Leukemia (CML): Current Treatment Strategies
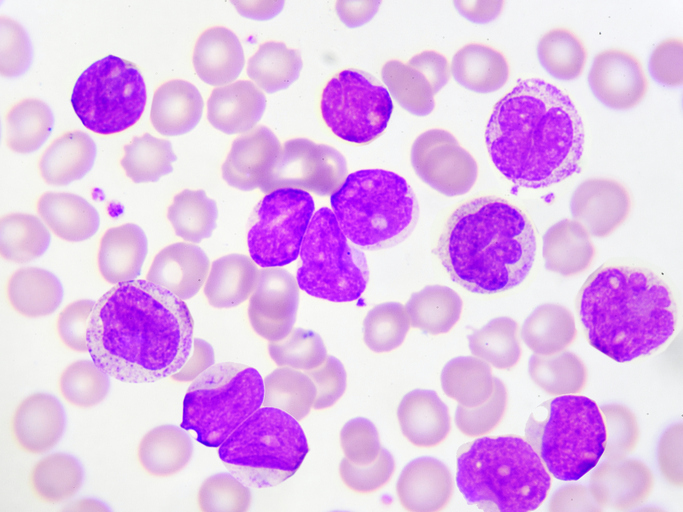
Chronic Myeloid Leukemia (CML) is a myeloproliferative neoplasm characterized by an overproduction of myeloid cells in the bone marrow. This overproduction leads to an increase in the number of these cells in the blood. CML is associated with a specific chromosomal abnormality called the Philadelphia chromosome, which results in the formation of the BCR-ABL1 fusion gene. This gene produces an abnormal tyrosine kinase protein that drives the uncontrolled growth of myeloid cells.
Understanding CML
CML is a clonal disorder of the hematopoietic stem cell. The disease typically progresses through three phases: chronic, accelerated, and blast crisis. The chronic phase is characterized by a relatively slow proliferation of myeloid cells, while the accelerated and blast crisis phases are marked by a more rapid and aggressive proliferation of immature cells.
Current Treatment Strategies
The treatment of CML has been revolutionized by the development of tyrosine kinase inhibitors (TKIs). These drugs specifically target the BCR-ABL1 protein, inhibiting its activity and effectively controlling the disease in most patients.
1. Tyrosine Kinase Inhibitors (TKIs)
TKIs are the standard first-line treatment for CML. They work by binding to the ATP-binding site of the BCR-ABL1 tyrosine kinase, preventing it from phosphorylating its substrates and thus inhibiting the downstream signaling pathways that drive cell proliferation and survival.
a. First-Generation TKIs: Imatinib
Imatinib was the first TKI developed and approved for the treatment of CML. It has demonstrated remarkable efficacy in inducing and maintaining remission in the chronic phase of the disease. Imatinib is generally well-tolerated, with common side effects including nausea, muscle cramps, edema, and skin rash.
b. Second-Generation TKIs: Dasatinib, Nilotinib, Bosutinib
Second-generation TKIs, such as dasatinib, nilotinib, and bosutinib, were developed to overcome resistance to imatinib and to achieve deeper and more rapid responses. These drugs are more potent inhibitors of the BCR-ABL1 kinase and have shown efficacy in patients who are resistant or intolerant to imatinib.
- Dasatinib: Dasatinib is a potent TKI that inhibits multiple tyrosine kinases, including BCR-ABL1, SRC family kinases, and others. It has been shown to induce faster and deeper responses compared to imatinib. Common side effects of dasatinib include pleural effusion, myelosuppression, and pulmonary arterial hypertension.
- Nilotinib: Nilotinib is another potent TKI that is more selective for BCR-ABL1 than imatinib. It has also demonstrated faster and deeper responses compared to imatinib. Common side effects of nilotinib include rash, pruritus, and cardiovascular events such as QT prolongation and peripheral arterial occlusive disease.
- Bosutinib: Bosutinib is a multi-kinase inhibitor that targets BCR-ABL1 and SRC family kinases. It has shown efficacy in patients resistant or intolerant to imatinib. Common side effects of bosutinib include diarrhea, nausea, and abdominal pain.
c. Third-Generation TKIs: Ponatinib
Ponatinib is a third-generation TKI that was developed to overcome resistance to other TKIs, particularly in patients with the T315I mutation in the BCR-ABL1 kinase domain. This mutation confers resistance to most TKIs except ponatinib. However, ponatinib is associated with a higher risk of arterial occlusive events, such as heart attack and stroke, and its use is typically reserved for patients with the T315I mutation or those who have failed multiple other TKIs.
2. Monitoring Treatment Response
Monitoring treatment response is crucial for optimizing outcomes in CML patients. Response is typically assessed using both hematologic and molecular criteria.
- Hematologic Response: This refers to the normalization of blood counts, including white blood cell count, platelet count, and hemoglobin level.
- Cytogenetic Response: This refers to the reduction or elimination of the Philadelphia chromosome in bone marrow cells. A complete cytogenetic response (CCyR) is defined as the absence of Ph-positive cells in the bone marrow.
- Molecular Response: This refers to the reduction in the level of BCR-ABL1 transcript in the blood, measured using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Molecular response is typically expressed as the ratio of BCR-ABL1 transcript levels to a control gene, such as ABL1. A major molecular response (MMR) is defined as a BCR-ABL1/ABL1 ratio of ≤0.1%. A deeper molecular response, such as MR4 (BCR-ABL1/ABL1 ≤0.01%) or MR4.5 (BCR-ABL1/ABL1 ≤0.0032%), is associated with a lower risk of disease progression and may allow for consideration of treatment discontinuation.
3. Treatment Discontinuation (TFR)
In patients who achieve a sustained deep molecular response (e.g., MR4.5) for at least two years, treatment discontinuation may be considered. This approach, known as treatment-free remission (TFR), has been shown to be successful in a significant proportion of patients, allowing them to remain in remission without ongoing TKI therapy. However, TFR is not suitable for all patients, and careful monitoring is essential to detect any signs of disease recurrence.
4. Allogeneic Stem Cell Transplantation
Allogeneic stem cell transplantation (allo-SCT) is a potentially curative treatment option for CML, but it is associated with significant risks and is typically reserved for patients who have failed TKI therapy or who are in the accelerated or blast crisis phase. Allo-SCT involves replacing the patient’s own bone marrow cells with healthy stem cells from a donor. This allows for the eradication of the leukemic cells and the establishment of a new, healthy immune system.
5. Management of TKI Resistance
Resistance to TKIs can develop in some patients due to various mechanisms, including mutations in the BCR-ABL1 kinase domain, amplification of the BCR-ABL1 gene, and upregulation of alternative signaling pathways. Management of TKI resistance typically involves switching to a different TKI, often a second- or third-generation agent. In some cases, allo-SCT may be considered.
6. Emerging Therapies
Several new therapies are being investigated for the treatment of CML, including:
- Asciminib: Asciminib is a novel TKI that binds to the myristoyl pocket of the ABL kinase, rather than the ATP-binding site. This unique mechanism of action allows asciminib to overcome resistance to other TKIs and has shown promising results in clinical trials.
- CAR T-cell therapy: Chimeric antigen receptor (CAR) T-cell therapy involves genetically modifying a patient’s own T cells to express a receptor that targets the BCR-ABL1 protein. These modified T cells can then recognize and kill CML cells. CAR T-cell therapy is currently being investigated in clinical trials for CML.
Conclusion
The treatment of CML has advanced significantly in recent years, with TKIs becoming the standard of care. These drugs have transformed CML from a life-threatening disease to a chronic condition that can be effectively managed in most patients. Monitoring treatment response is crucial for optimizing outcomes, and treatment discontinuation may be considered in patients who achieve a sustained deep molecular response. Allo-SCT remains an important treatment option for patients who fail TKI therapy, and emerging therapies such as asciminib and CAR T-cell therapy hold promise for further improving outcomes in CML.
Disclaimer: This article is for informational purposes only and does not constitute medical advice. Always consult with a qualified healthcare professional for diagnosis and treatment of CML.







Leave a Reply